Since FDA approved the first smart pill for use in the United States last year, several companies have come forward with similar pills and trying to get FDA approval for their products.
Abilify MyCite
The smart pill that FDA approved is called Abilify MyCite. This tiny pill has a drug and an ingestible sensor. The sensor gets activated when it comes into contact with stomach fluid to detect when the pill has been taken. The data is then transmitted to a wearable patch that eventually conveys the information to a paired smartphone app. Doctors and caregivers, with the patient’s consent, can then access the data via a web portal.
Although the pill got FDA approval, it comes with numerous warnings.
“It is important to note that Abilify MyCite’s prescribing information (labeling) notes that the ability of the product to improve patient compliance with their treatment regimen has not been shown. Abilify MyCite should not be used to track drug ingestion in “real-time” or during an emergency because detection may be delayed or may not occur,” the FDA said in a statement.
Read more Combining Wearable Tech and AI Could Help Predict Onset of Diseases
The idea of a digital pill that registers when it has been taken is novel, but as the FDA notes, there is no proof that it actually increases the probability that patients who do not usually follow routine when taking medications will follow their prescribed course.
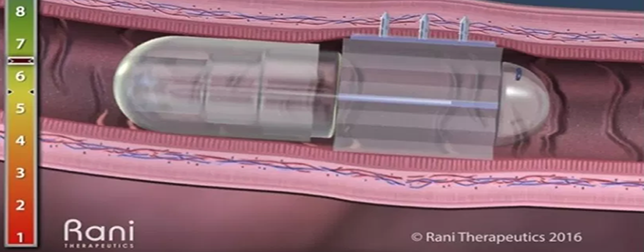
Rani Therapeutics
Rani Therapeutics has developed an innovative approach for the oral delivery of large drug molecules including proteins, peptides and antibodies.
The pill delivers an intestinal injection without exposing the drug to digestive enzymes. The patient takes what seems to be an ordinary capsule, but the “robotic” pill is a sophisticated device which incorporates a number of innovations, enabling it to navigate through the stomach and enter the small intestine. The Rani Pill™ goes through a transformation and positions itself to inject the drug into the intestinal wall.
Mir Imran, Chairman & CEO of Rani Therapeutics, who will be speaking at the WT | Wearable Technologies Conference in San Francisco on July 11-12, said:
“We are tackling one of the greatest challenges in drug delivery: converting injectable drugs into pills. By replacing painful injections with pain-free pills, we have the potential to improve the quality of life for millions of patients and this is what motivates us.”

ID-Cap
The ID-Cap by etectRx is a standard hard gelatin or HPMC capsule with an embedded ingestible wireless sensor – the ID-Tag™. When a person swallows ID-Cap, the ID-Tag uses etectRx’s proprietary communications technology to transmit a very low power digital message from within the patient’s stomach. The smart pill is:
Patient friendly – It looks and tastes just like an ordinary capsule.
Communicates every dose – One or two-way communication between the ID-Tag and a wearable device called “the Reader” ensures every ingestion is detected. The ID-Tag communications technology has demonstrated high reliability in multiple clinical studies.
Self-powered – The patient’s own stomach fluid powers the ID-Tag.
Leaves no trace – After the capsule dissolves, the ultra-thin, flexible sensor is discharged naturally through the patient’s GI tract.
A special note: Harry Travis, President & CEO of etectRx, will be speaking at the WT | Wearable Technologies Conference in San Francisco on July 11-12.