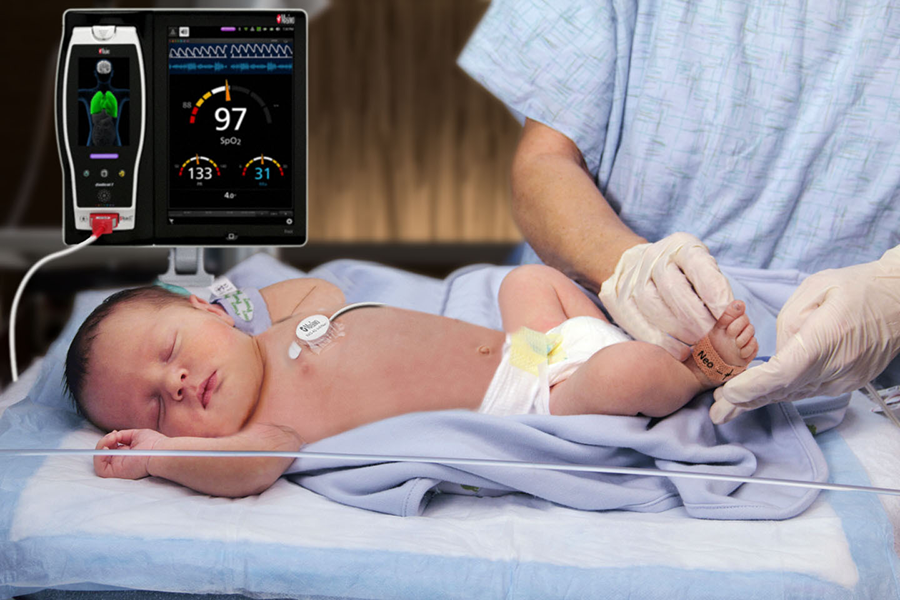
Masimo, the Irvine, CA-based American manufacturer of noninvasive patient monitoring technologies, announced the FDA approval of its RAS-45, an acoustic respiration sensor for rainbow Acoustic Monitoring® (RAM®), for infant and neonatal patients. Previously, RAM could be used with RAS-125c and RAS-45 sensors to monitor adult patients weighing more than 10 kgs. Now, with the FDA cleared sensor, RAM would be able to monitor patients of all sizes, including neonates, in the United States.
Read more Bloomlife Pregnancy Tracker Keeps You and Your Baby Safe by Monitoring Contractions
RAM uses an adhesive Respiratory Acoustic Sensor (RAS) with an integrated acoustic transducer, the RAS-45 and RAS-125c to noninvasively and continuously measure respiration rate of adults and infants. Using acoustic signal processing that leverages Masimo Signal Extraction Technology® (SET®), the respiratory signal is separated and processed to display continuous respiration rate (RRa®) and an acoustic respiration waveform, a visualization of the vibrations caused by the patient’s airflow. The doctors can also listen to the sound of a patient’s breathing at bedside or remotely.
With the clearance for newborns and neonates, RRa’s accuracy range has been expanded up to 120 breaths per minute, while still providing accuracy of +/- 1 breath per minute, facilitating accurate measurement of the higher respiratory rates common in this population.

The sensor itself is very small, slightly larger than a nickel. Similarly, it weighs so little (13 grams) that its presence may be barely noticeable, and features an adhesive that is transparent, light, and flexible. The size, weight, and adhesive advantages make it particularly suitable for the smaller stature and delicate skin of infants and neonates.
In a study involving 40 patients, the RRA has been found to be accurate, reliable, easy to tolerate and to enhance patient compliance with respiration monitoring.
“From the beginning, we have focused our R&D on neonates and children for many reasons, including our belief that helping clinicians care for children will provide more benefit to society. RAM harnesses the power of our breakthrough signal processing and sensor technology and applies it to a measurement that has either been unreliable or difficult to use, respiration measurement, the third vital sign,” said Joe Kiani, Founder and CEO of Masimo.
Read more Isansys the World’s Most Advanced Wireless Patient Monitoring Platform Gets Key US Patent
RAM is available on most rainbow SET™-ready platforms. Continuous monitoring of respiration rate can be helpful in cases such as sedation-based procedures and post-surgical patients receiving patient-controlled analgesia for pain management.