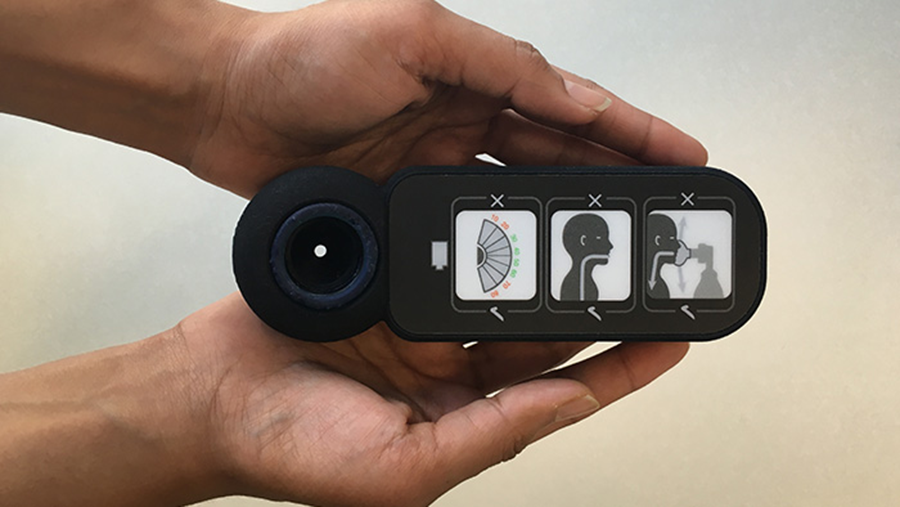
Philips, the Dutch multinational technology company, in collaboration with the researchers at Massachusetts General Hospital, Mbarara University of Science and Technology in Uganda, and MIT, has developed ‘Augmented Infant Resuscitator (AIR)’ – a device for conventional neonatal bag-valve-mask (BVM) resuscitators that helps care givers to effectively resuscitate asphyxiated newborns. The AIR can be attached to almost all manual BVM resuscitators to measure and help regulate the aiflow delivered to the baby.
Related Masimo’s Rainbow Acoustic Monitoring RAS-45 Breathing Sensor for Babies Gets FDA Clearance
Perinatal asphyxia or birth asphyxia is a medical condition resulting from prolonged deprivation of oxygen to a newborn that lasts long enough during the birth process to cause physical harm, usually to the brain. Hypoxic damage can occur to most of the infant’s organs (heart, lungs, liver, gut, kidneys), but brain damage is of most concern and perhaps the least likely to quickly or completely heal. Globally, birth asphyxia causes more than 800,000 neonatal deaths annually, and over one million potentially preventable reported stillbirths. Effective resuscitation could reduce birth asphyxia related neonatal deaths by 30%, and deaths from prematurity by 10%.
Currently available manual resuscitators are usually proven to be unsuccessful, especially in parts of the world that are underserved in terms of healthcare, thus do not have access to expensive equipment. Doctors may give too much air or too little, too fast or too slow, with peak airway pressures increasing to a very high level, often harming the baby in the process.

The AIR device measures ventilation flow and pressure to monitor the quality of ventilation, and provides intuitive visual feedback on common ventilation errors, including inadequate face-mask seal, obstructed airway, incorrect ventilation rate, and harsh breaths that can damage the baby’s airways. AIR also records performance for future feedback, improving the training of healthcare professionals by identifying persistent gaps in technique.
“At Philips, we aim to improve people’s health through meaningful innovations,” said Arman Voskerchyan, Business Leader for Therapeutic Care at Philips. “Our mission is to improve the lives of three billion people a year by 2025. By combining our expertise in respiratory care and resuscitation with the strengths of global health innovators like the AIR team at CAMTech, we aim to drive and scale innovative solutions that bridge societal divides in healthcare to reach underserved populations, and thereby addressing the United Nations Sustainable Development Goal 3.”
The AIR device prototype was first developed in 2012 by Dr. Kristian Olson, Director of CAMTech at Massachusetts General Hospital Global Health, Dr. Data Santorino, CAMTech Uganda Country Manager, and Dr. Kevin Cedrone, a lecturer at the Massachusetts Institute of Technology (MIT), at a CAMTech Hack-a-thon with MIT at Massachusetts General Hospital. An initial grant from CAMTech enabled support by Saving Lives at Birth (SLAB) to further develop the device.
The device is expected to be available in limited volume in selected markets prior to scaling up availability in low- and middle-income countries.